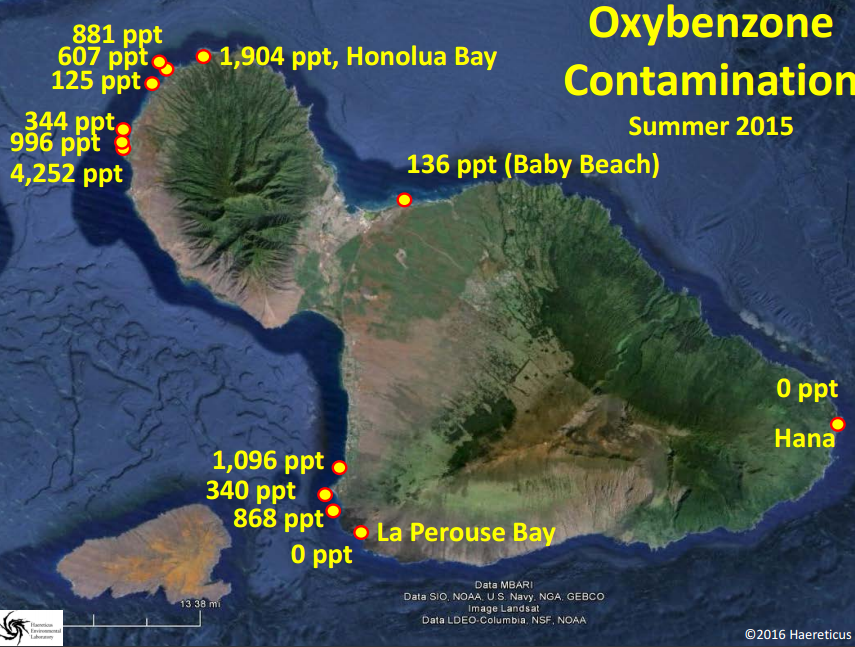
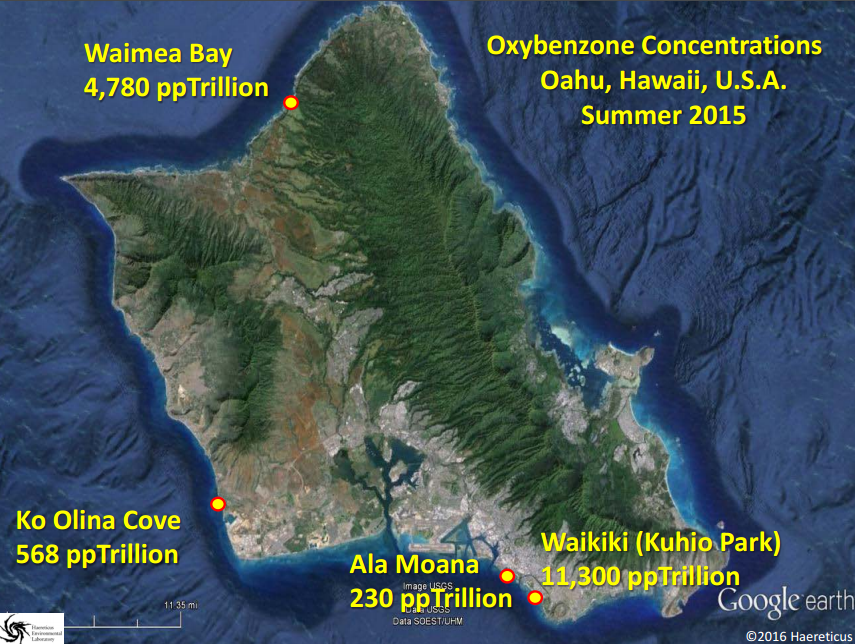
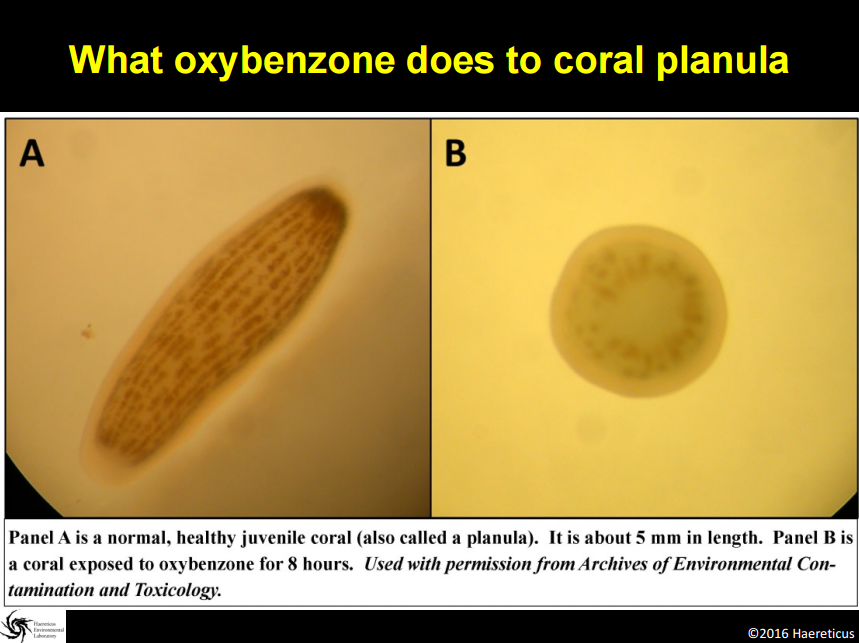
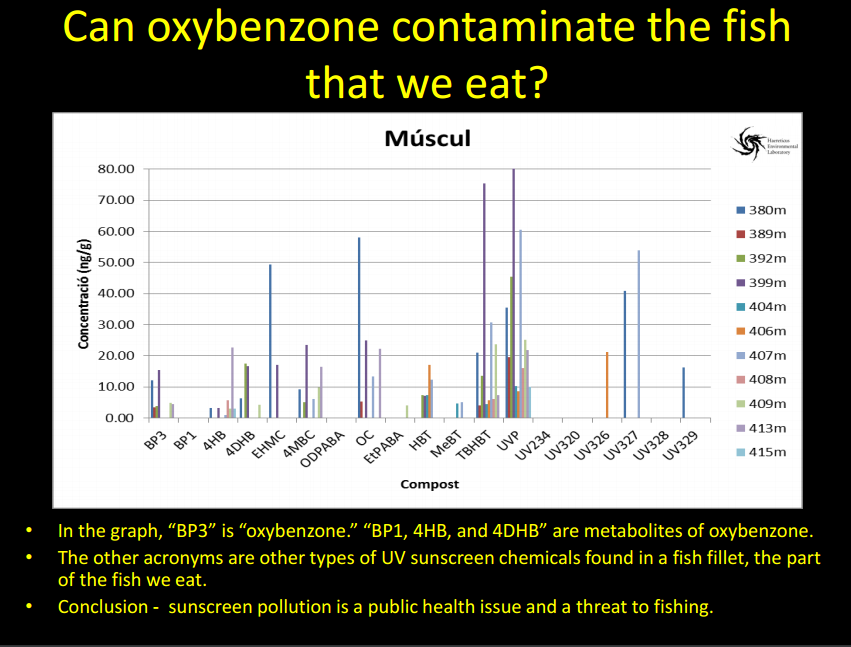
Support an Oxybenzone Ban in Hawaii: Stop and Check!...Your Sunscreen Might be Killing the Reef!7/22/2016 7/22/16 We are here this morning with Craig Downs, the Executive Director of the Haereticus Environmental Laboratory based in Clifford, Virginia. Dr. Downs and his team recently published a study in the 'Archives of Environmental Contamination and Toxicology' which examined the link between oxybenzone - an ingredient commonly found in sunscreens - and coral bleaching. Dr. Downs who is graduate of the University of Hawaii-Manoa, returned to the islands for the International Coral Reef Symposium in June where he explained his research and advocated for a ban on oxybenzone in Hawaii. In response to his presentation, State Senator Will Espero (Dist. 19) announced that he will introduce legislation for Hawai'i to ban sunscreen with oxybenzone beginning in 2018. We here at HOA were lucky enough to get a chance to ask Dr. Downs some questions about his research and are sharing his responses with you in hopes that we can all help educate one another about the dangers of oxybenzone, take seriously the need to swap out our current sunscreens for more reef friendly ones, and do all we can as citizens to encourage our elected representatives to support a ban on these chemicals which are dramatically harming our fragile coral reefs. HOA: Good morning Dr. Downs and thanks so much again for taking the time. Could you please describe for us what oxybenzone is and how it is damaging our reefs? Dr. Downs: Oxybenzone is an aromatic, hydrocarbon-based ultraviolet-light absorbing chemical. It is in the benzophenone-family of chemicals, which all absorb UV-light. It is used as a primary ingredient in many sunscreen lotions and sprays. It is readily absorbed through the skin of humans and other marine mammals, as well as by coral tissue. From a physiological perspective, oxybenzone can cause pathological damage a number of ways. For corals, it (1) can induce coral bleaching, and is exacerbated by intensity and composition of light, (2) it causes coral planula (juvenile form of coral) to become radically and quickly deformed, (3) it causes damage to DNA, especially in the presence of light, and (4) it is an endocrine disruptor, altering developmental cues of skeletal-genesis. The cosmetic company, L’Oreal, independently confirmed our study that oxybenzone (and avobenzone) caused damage to the coral’s zooxanthellae’s photosynthetic process – one of the first molecular and cellular steps in coral bleaching. In fish, studies to date have not looked for the gross morbidity that we see with corals, but they do see significant changes to a number of physiological systems – it is both a hormonal endocrine disruptor, as well as neurological/behavioral disruptor. Exposure to oxybenzone can induce feminization in male fish, or potentially inhibit a process called sequential hermaphroditism – an important reproductive process in many marine fish species. We are particularly concerned with species such as parrot fish, clown fish, gobies, moray eels and wrasses, because we know for many of these species, population survival is critical for this process to remain healthy. In females, exposure to oxybenzone causes reduced egg production, as well as causes egg-protein production in adult males or in sexually immature juveniles. A recent study coming out of laboratory in Taiwan showed that oxybenzone significantly altered fish behavior, making males less territorially aggressive. A study conducted by Paredes and coworkers (2014, Chemosphere 104:44-50) showed that oxybenzone exposure inhibit growth in both juvenile shrimp and bivalves. Work from our lab confirmed Paredes finding of growth inhibition of larval shrimp, and that toxicity was light dependent (you get different results when running the experiment under “normal laboratory light”, which is about 100 times less sunlight than what you see at noon, and when you expose the larval shrimp to oxybenzone and 20% sunlight). Unpublished work from our laboratory indicates that oxybenzone in the presence of natural sunlight is highly toxic to sea urchin sperm, inhibits fertilization of sea urchin gametes, and causes deformity in developing sea urchin embryos and early-stage juveniles (pluteus stage). When we take a “step-back” in perspective, this organismal toxicity, when operating in the wild, can have drasticecological consequences. We began our investigation into oxybenzone in response to a larger study of why we were seeing very little to no coral recruitment on many reefs, especially in the Caribbean. It couldn’t be explained by climate change, because in one bay with almost no swimmers on a daily basis, there would be a reef area with a lot of recruits of baby coral, and then in an adjacent bay, where swimmer density could reach 3,000 people at a time, there would be no coral recruits. We also noticed that the corals in the bay with swimmers were all sterile, yet the corals in the adjacent bay were abundant in sperm and egg packets. It wasn’t just coral that exhibited this diseased state. Sea urchins in these same two bays (especially Tripneustesspecies) exhibited a similar reproductive condition as the corals. Sea urchins from the healthier reef had 25 to 45 times more sperm and eggs per individual compared to the unhealthier reef. When we tried fertilizing sperm and eggs from individuals from the same sites, the healthier site had a fertilization ratio of 1 egg to 250 sperm, while the unhealthier site had a fertilization ratio of 1 egg to 35,000 sperm. The embryos from the healthy site developed normally, while embryos from the unhealthy site would develop abnormally, and even cease development by the fifth cell division. I thought it a sampling fluke, but we noticed that there was definite difference in the number of females to males (close to 4:1 females to males). We recently just heard that this skewing of sea urchin sex ratio is not an illusion, but something that other scientists are seeing in other parts of the world (Bronstein et al (2016) Reproduction of the long-spined sea urchin Diadema setosum in the Gulf of Aqaba - implications for the use of gonad-indexes. Sci. Rep. 6, 29569; doi: 10.1038/srep29569). This disease phenomenon also exists in Maui, Hawaii. We inadvertently had to sample Tripneustes for gametes from La Perouse to Kapalua Bay, and found almost no viable gametes or embryos except at a site in La Perouse that is largely unused by surfers or snorkelers (water is too rough). We were hoping that Olowalu would have healthy specimens, but a lot of that reef area was destroyed by a sedimentation event. We noticed at a number of sites, that the Collector’s Urchins we collected had way more females than males, and the females did not produce very many eggs (e.g., Makena Landing Beach Park, Napili Bay). We encourage other citizen scientists to run the experiment and monitoring to verify if our one-time observation has merit as a health condition for these sea urchin meta-populations. Saddle Wrasse, Thalassoma duperrey feeding on sea urchin Tripneustes gratilla in Kona Source: Brocken Inaglory. Date: 11 May 2010. We use the term “Coral Reef Zombies” to describe this community-wide dysfunction. From an untrained eye and shifting baseline expectations, the corals and other reef organisms that you see at a location are present. But… demographically, that population/community is dead, because no future generations will arise. The adults are not producing a healthy next-generation, and there is no “next-generation” recruiting into that area. It is only a matter time before the reef disappears. Oxybenzone could be one etiological factor (causal) that gives rises to these “zombies.” It is almost for certain that it is not the only pollution factor that can contribute to zombies. A lot of folks talk about managing ecological resiliency to climate change. One of the most important factors that degrades coral reef resiliency to climate change is pollution. And we have shown that oxybenzone degrades that resiliency by compromising the physiology of a coral reef’s keystone species. If you want to increase the stamina of near-shore reefs to heat stress (one of the factors associated with climate change events), then you need to remove oxybenzone. Oxybenzone is a photo-toxicant, it induces photo-oxidative stress in the presence of sunlight, and especially ultraviolet light. It exacerbates the oxidative stress load onto a coral. Unpublished work from our lab showed that coral (Pocillopora damicornis, which used to be plentiful in Hawaiian waters and now it is really difficult to find) when induced to bleach by heat stress (30ºC), the coral will bleach and “eat” its zooxanthellae algae, but have a high probability of recovering and surviving from the bleaching event. On the other hand, when you add 300 parts per trillion oxybenzone to the same heat stress event, it kills >70% of the coral in less than 8 days. It does it by causing a cellular-level process called necrosis – the coral cells that contain the zooxanthellae don’t eat the algae, they just die. This results in a chain reaction to nearby cells, until you get tissue-level death. The coral just can’t survive this tissue necrosis. HOA: Your study focused on the Virgin Islands and Hawaii. Can you describe for us what the concentrations of oxybenzone was in each location and what those concentrations mean for the health of the associated reefs? Dr. Downs: In areas where there are a lot of swimmers per day (more than a hundred), we found concentrations of oxybenzone in the parts per billion. At Trunk Bay in the U.S. Virgin Islands, where you can have several hundred swimmers to 2,000 swimmers at a time, we saw a level in the parts per million. In Maui, of the 13 or more sites we sample, many sites were between >100 parts per trillion and 1,000 parts per trillion. The beach area south of Blackrock in front of the Sheraton in Maui, we saw levels at 4,252 parts per trillion. Even when water conditions are really bad, and no one has been in the water for most (if not all) of the day, levels of oxybenzone at a site can still persist – “Baby Beach” in Spreckelsville, Maui had a level of 136 parts per trillion with no one in the water all day or sitting on the beach. On Oahu, we saw levels at 4,780 parts per trillion at Waimea Bay during a summer sampling. The most southern “cove’ of Ko Olina Cove had levels of 568 parts per trillion, with less than 16 people in the water before 10 am. Kuhio Park in Waikiki had 11,000 parts per trillion. Something to consider - though these levels fluctuate in response to tides, seasons, and density of swimmers at a site for the day, oxybenzone is being refreshed every single day into these waters. Coral reefs don’t have a chance in avoiding this exposure. HOA: Are these levels to be considered threatening to coral reef health? Dr. Downs: Yes! Coral cells die when exposed to oxybenzone and 20% normal sunlight in less than 4 hours when exposed to just 72 parts per trillion oxybenzone. Coral planula will significantly “bleach” when exposed to 2 parts per billion oxybenzone within just 8 hours. Adult coral will bleach as low as 400 parts per trillion when exposed to oxybenzone for 5 days (at 26.7ºC). HOA: Does oxybenzone also harm other marine wildlife in addition to fish, sea urchins, and the coral reef? Dr. Downs: Oxybenzone and other chemicals found in personal care products is a relatively new and unexplored field of environmental pollution. Not much is known about it, because there is almost no research being done on the topic. Currently, the best research on the topic is happening outside of U.S. institutions, such as research and government labs in Spain, China, and Greece. The Spanish and Brazilians have recently shown that dolphins have relatively high loads of oxybenzone and its metabolites, and that oxybenzone can be transferred from mother to fetus/infant via umbilical cord and breast milk. A forthcoming scientific paper from the Spanish shows that a number of threatened migratory birds in Spanish nature reserves are accumulating oxybenzone (perhaps from eating contaminated fish) and the oxybenzone is accumulating to high levels in their eggs. Who knows what oxybenzone is doing to monk seals, sea turtle nesting sites (especially since aerosol spray sunscreens can readily contaminate beach sand, and this would surely percolate down to the eggs), and even to Humpback and Pilot whales that partake of Hawaiian waters? HOA: So, sunscreen pollution is not just harming our environment, it's working its way into the food chain and making its way to our tables? Dr. Downs: This is a worrisome possibility. Again, the Spanish and the Brazilians published separate studies examining the levels of about 32 different sunscreens chemicals in the gills, liver, and muscle fillet of freshwater and estuarine fish. What was interesting about these studies is that they showed that fish livers will metabolize oxybenzone into benzophenone-1; a much more potent estrogenic endocrine disruptor. They found concentrations of about 15 parts per billion oxybenzone in fish fillets, while octinoxate concentrations in the same fillet samples ranged from 15-50 parts per billion. Barcelo et al (2015) Accumulation of UV filters in fish and sediments from Iberian river basins. Science of the Total Environment 518-519:518-525 Molins-Delgado et al (2015) Analyzing personal care products in biota by liquid chromatography – atmospheric pressure photoionization-mass spectrometry. Society of Environmental Toxicology and Chemistry. A very preliminary investigation in Hawaii indicates similar results in Hawaiian-caught fish. HOA: Part of your study was focused on aerosol sunscreen and how this method of application is especially damaging. Why is aerosol can-based sunscreen particularly harmful? Dr. Downs: Besides being an annoyance to everyone around the person spraying it, and the possibility that it may significantly threaten the health of infants and children if they breathe in the mist (see links below), most people can attest that when someone uses these sprays on the beach, much of it goes off into the air, and not on the person. That non-target misting with all those chemicals goes somewhere, and that somewhere is usually the beach sand. When the high tide encroaches onto the beach where 6 hours before, people were sitting on that sand spraying themselves down, those chemicals are “liberated” from the sand and enter into the water column. The highest concentrations of oxybenzone in the near-shore water column were about 2 hours after the high-tide mark. They were lowest at the low-tide mark. http://www.consumerreports.org/cro/news/2011/07/don-t-spray-sunscreens-on-kids-at-least-for-now/index.htmhttp://www.huffingtonpost.com/2014/07/07/spray-sunscreen-safety-kids_n_5564533.html
HOA: What are some alternatives to oxybenzone sunscreens? Dr. Downs: There are a number of other chemicals that work as UV absorbers. There are 17 chemicals approved by the U.S. FDA that are allowed to be included into U.S. sunscreen products. Some of these chemicals are just as toxic as oxybenzone (or more so, such as octinoxate), while others seem to be less toxic. To be honest, there just isn’t a whole lot of ecotoxicological data on ANY of these chemicals, let alone toxicological data on mammals and humans. There was a recent report by a Danish group showing that eight of the 17 UV filters approved for use in the U.S. disrupted human sperm function: https://www.sciencedaily.com/releases/2016/04/160401111847.htm http://www.cnn.com/2016/04/14/health/sunscreen-sperm-male-fertility/ I can say that from our laboratory studies, nanotized (below 100 nanometer) zinc oxide particles caused coral bleaching. I saw a poster at the 2016 International Coral Reef Symposium where a scientific group in Monaco was collaborating with L’Oreal (the cosmetic company), and they too saw coral bleaching with zinc oxide (I think it was nanotized ZnO). We did not see coral bleaching with non-nanotized (above 350 nanometers) zinc oxide that was coated with silica. The silica supposedly prevents the transfer of high-energy electrons to dioxygen, stopping the formation of radical oxygen species and oxidative stress. For the time being, one suggestion is to consider using products that lack certain chemicals as ingredients, such as the chemicals listed in the following websites: http://www.marinesafe.org/science-and-data/marine-pollutants-identified-by-science/ http://www.xcaret.com/faqs.php http://www.xelha.com/xelha-all-inclusive.php Sun clothes are also an effective strategy for sun protection, and reducing the amount of sunscreen cosmetic product that you use. By limiting the use of the sunscreen to the back of our necks, face, and back of your hands, you can reduce sunscreen environmental contamination by as much as 90%, compared to lathering it all the entire body. HOA: What other actions can people immediately take to bring about change? Dr. Downs: There are three major things an individual can do, and these actions can be tremendously magnified if done as a local group. Consumer Activist – Manufacturers respond to consumer demands. Even major retail distributors respond to consumer demands. Just this week, Wal-Mart is requesting of its suppliers to stop using eight chemicals in their products. Some of these chemicals are in many sunscreen products as preservatives. Just look at the ingredient labels. http://www.bloomberg.com/news/articles/2016-07-20/wal-mart-asks-suppliers-to-remove-eight-chemicals-from-products
Citizen Activist – Coral reefs suffer from the “Tragedy of the Commons”, everyone expects someone else to be responsible and take care of our coral reefs. Usually, that someone else is “the government.” The government will usually respond if its citizens demand of them to do something effective.
Community Activist – Businesses, churches, temples, and mosques, as well as other non-profit organizations can be made aware of this issue, and encouraged to develop policies and relationships that help mitigate sunscreen pollution along Hawaii’s coast. For example, individuals can make friendly overtures to resorts and hotels to post banners in their lobby shops or near concession stands by the beach informing guests to be aware of the issue. Resorts can follow a Wal-Mart policy, and request products in their lobby shops to NOT contain any of the threatening chemicals. Individuals can petition local Wal-Mart, Costco, Target, etc to not stock sunscreen products that contain oxybenzone/octinoxate. Engage the Waikiki Aquarium and Maui Ocean Center to consider putting up a conservation display on the issue
HOA: Thanks so much for taking the time to respond to our questions and for all of the great work you and your team do at the Haereticus Environmental Laboratory to raise awareness of the damaging effects of oxybenzone sunscreens. Here's hoping that our representatives are successfully able to pass a ban on oxybenzone in Hawaii and that in the meantime our readers make the decision to alter their behaviors and take the time to reach out to companies that manufacture sunscreens as well as their elected representatives to advocate for alternatives to these damaging products. Mahalo nui loa and Aloha, HOA P.S. A very special thanks to Bob Kern, Vice President of Friends of Hanauma Bay, for the introduction! Notes:
2 Comments
6/30/2018 01:02:46 am
It is unfortunate that sunscreen is hurting the corals and sea life, for I am one of the many people who love going to the beach, but is easily burned by the sun’s rays. I apparently need more melatonin on my skin to protect me. However, I cannot in good conscience use sunscreen any longer after learning that it is damaging fish’ homes, not to mention the fish that live there. It would disastrous to marine life if the corals are destroyed. So, maybe next time I go to the beach, I’ll just bring an umbrella and stay under the shade.
Reply
12/12/2018 12:05:27 am
Thank you for an additional information regarding sunscreen and coral reefs. I have no idea on what had happened whenever people put sun screen then go in the water. I am one of those people who does this, but I had no idea at all. At least now, I have my idea. I will tell my friends regarding this matter. We all love going into beaches and giving this knowledge to them would be really helpful. I guess that I will not put too much sunscreen the next time I go in the water.
Reply
Leave a Reply. |
|